Introduction
Gestational Diabetes Mellitus is defined as carbohydrate intolerance which onset or first detection is made during pregnancy, associated with diverse maternal-and fetal adversities (Kc et al., 2015; Billionnet et al., 2017; Kautzky-Willer et al., 2019). Since 2010, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) released their recommendations for new diagnostic values based on the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO study et al., 2008) and have been gradually adopted by the World Health Organization (WHO), the Australian Society for Diabetes in Pregnancy (ADIPS), the American Diabetes Association (ADA), the American Association of Clinical Endocrinologists (AACE) and the International Federation of Gynecologists and Obstetricians (FIGO).
GDM is diagnosed by the IADPSG criteria when fasting glucose values are >92 mg/dL, and/or if glycemia values in the first hour are >180 mg/dL and/or in the second hour are >153 mg/dL after a glucose load of 75-g (Gorban de Lapertosa et al., 2020; IADPSG et al., 2010). The National Institute for Health and Clinical Excellence (NICE) updated their criteria, setting up the levels for fasting glucose levels of >100 mg/dL, as well as >140 mg/dL at 2 hours after a glucose load of 75-g (Johns et al., 2018). The differences of subtracting the values of IADPSG against NICE at the point of fasting glucose and 2hr after a glucose load of 75-g, imply that the diagnosis of GDM by NICE is made with a fasting glucose level 8 mg/dL higher than with IADPSG, but with 13 mg/dL less than IADPSG at 2 hours after a glucose load of 75-g, which could mean that these are two types of patients who have different pathophysiological problems: the first could have more resistance to insulin and the second a problem based on defects in insulin secretion.
The Latin American Diabetes Association (ALAD) decided to adhere to the NICE criteria since the IADPSG criteria increase the number of women diagnosed with GDM and thus, increase the work and economic load in health systems, in addition to the growth of anxiety and stress levels of patients who would be healthy with other criteria, without an improvement until now in the cost-effectiveness of the intervention or the number of outcomes (Salzberg et al., 2016). However, there is also the other scenario in which NICE criteria leads to misclassification of GDM patients as healthy pregnant women; therefore, the long-term costs of not treating these women and their newborns could justify the expenses implied by the IADPSG criteria (Brown et al., 2017; Djelmis et al., 2016).
The aim of this study was to compare the risk factors and maternal-fetal outcomes associated with GDM patients among the IADPSG and NICE criteria to find the most suitable for the detection of GDM patients in Mexican population.
1. Patients and methods
1.1. Design of the study and cohort
A prospective cohort study was performed in 485 patients attended at the General Regional Hospital “Lic. Ignacio García Téllez” from the Mexican Institute of Social Security (IMSS) in Madero City, Tamaulipas; in a period between August 2nd, 2016 to April 14th, 2020. The Committee of Ethics from the General Regional Hospital “Lic. Ignacio García Téllez” approved the study. All patients signed an informed consent for their inclusion in the study.
1.2. Diagnosis of GDM and Oral Glucose Tolerance Test (OGTT)
The diagnosis of GDM was made according to the IADPSG criteria, a positive measurement of any of the following values: fasting glucose level > 92 mg/dL, glycemia after OGTT 75-g of >180 mg/dL after 1 hour and > 153 mg/dL after 2 hours. For the diagnosis of GDM according to the NICE criteria, a positive measurement of a fasting glucose level > 100 mg/dL and glycemia after OGTT 75-g of >140 mg/dL after 2 hours. For both cases, the OGTT was performed with the ingestion of 75-g of anhydrous glucose in 377 cc of water at room temperature and ingested in no more than 5 minutes, taking glucose measurements at the time points showed for each criterion. Patients who had a diagnosis of Type 1 or Type 2 Diabetes Mellitus (T1DM, T2DM) before pregnancy or patients whose clinical history was incomplete were excluded. We refer to the healthy pregnant women, as the ones having a GDM negative diagnosis, although this does not discard other potential comorbidities. Also, we refer to the final diagnosis as the diagnosis of GDM performed with the fasting glucose levels or glycemia at OGTT 1h or OGTT 2h, according to cut-off values of IADPSG or NICE criteria previously mentioned.
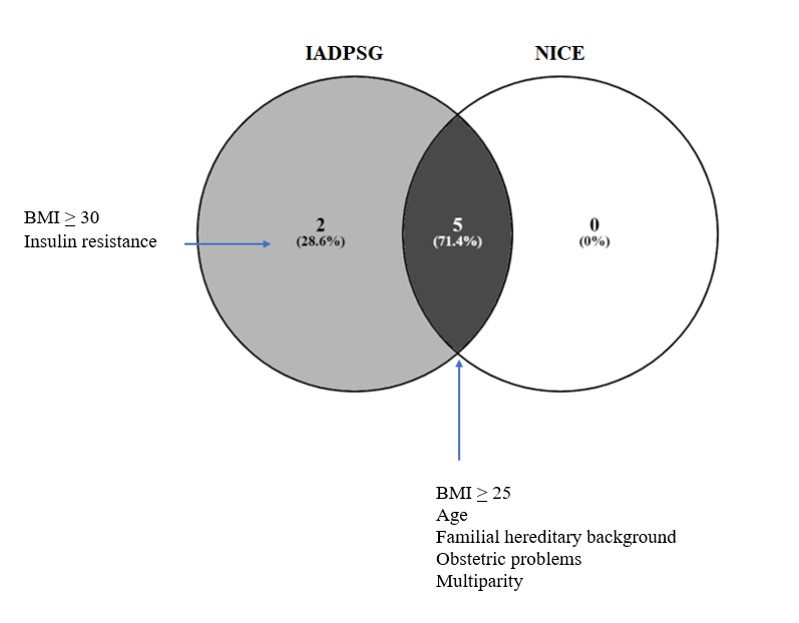
1.3. Variables evaluated
The comparison of the following variables was made: age, body mass index (BMI), week of gestation at the time of GDM diagnosis (WG), glycated hemoglobin, cholesterol and triglycerides. In the case of newborns, the variables evaluated were height, weight and week of gestation at birth. The patients were classified according to BMI as underweight (BMI <18.5), normal weight (BMI from 18.5 to 24.9), overweight (BMI from 25 to 29.9), obese (BMI from 30 to 34.99), and severe obese (BMI > 35.0) (Kim et al., 2010; Torloni et al., 2009). The cholesterol and triglyceride index of the cohort was analyzed, considering as altered values as > 200 mg/dL and >150 mg/dL, respectively. The risk factors included: age over 30 years; patients with a BMI of 25 or more in early pregnancy; hereditary family history of diabetes mellitus; presence of obstetric problems; presence of insulin resistance; weight gain greater than 20 kilograms; presence of arterial hypertension; presence of hyperlipidemia and multiparity. Insulin resistance refers to the presence of associated pathologies as acanthosis and/or polycystic ovary syndrome. The analyzed outcomes were: preeclampsia; presence of urinary tract infection, cervicovaginitis or both; premature rupture of membranes; induction of labor; abortion; form of birth (dystocic or eutocic pregnancy, caesarean section); twin pregnancy; hypoglycemia; respiratory distress syndrome; acute fetal distress; oxygen requirement; requirement of intubation; presence of jaundice; presence of hydramnios and macrosomia.
1.4. Statistical Analysis
The statistical program SPSS (IBM, version 20) was used for comparative studies. The variables were presented with descriptive statistics such as frequency, percentage, mean and SD. The Kolmogorov-Smirnov test was employed to assess normality in continuous variables. For those that followed a normal distribution, Student’s t-test was selected; for those that did not, the U Mann-Whitney test was applied. Contingency tables, Fisher’s exact test, and χ2 were used for discrete variables. Odds Ratios (ORs) and 95% confidence intervals were calculated. A value of p <0.05 was considered statistically significant. The construction of graphs, Forest plots and Heat maps was performed with program GraphPad Prism version 8.0.2. Venn diagrams were made with the program Venny available in https://bioinfogp.cnb.csic.es/tools/venny/.
2. Results
2.1. Characteristics of the Mexican cohort diagnosed with GDM
216 pregnant women received a diagnosis of GDM with a prevalence of 44.5% and 269 pregnant women were classified as healthy according to IADPSG; whilst 182 had a GDM diagnosis with a prevalence of 37.5% and 303 pregnant women were classified as healthy according to NICE. We found significant differences among GDM patients and healthy women (p <0.05) in BMI, age, glycated hemoglobin and triglycerides. Between patients diagnosed with GDM by both criteria we did not found significant differences: mean age was 32 years, BMI 32 kg/m2 with a 92% prevalence of overweight and obesity, glycosylated hemoglobin 5.6% and elevated levels of cholesterol and triglycerides (Table 1). The offspring of GDM patients diagnosed by both criteria presented quite similar values for weight, height and week of gestation at the time of birth (Table S1)
Table 1
Characteristics of patients with GDM by IADPS and NICE criteria in a Mexican population
|
Characteristics |
IADPSG |
NICE |
Difference among diagnostic criteria |
||||||
|
Healthy |
GDM |
Healthy |
GDM |
||||||
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
|
|
|
Age (years) |
29 |
6.0 |
31.9 |
5.3 |
29.3 |
5.7 |
32.07 |
5.6 |
NS, p=0.7 |
|
BMI |
30.4 |
6.7 |
32.27 |
5.4 |
30.8 |
6.8 |
32.08 |
5.08 |
NS, p=0.7 |
|
WG |
26.9 |
6.6 |
26.7 |
6.3 |
26.7 |
6.6 |
26.8 |
6.3 |
NS, p=0.8 |
|
Glycosylated hemoglobin (%) |
5.1 |
0.4 |
5.6 |
0.5 |
5.2 |
0.4 |
5.6 |
0.5 |
NS, p=0.9 |
|
Cholesterol (mg/dL) * |
222.8 |
43.7 |
213.6 |
45.2 |
220.9 |
42.6 |
215.5 |
48.6 |
NS, p=0.7 |
|
Altered Cholesterol (%) * |
70.6 |
- |
58.1 |
- |
69.0 |
- |
58.5 |
- |
- |
|
Triglycerides(mg/dL) * |
203.5 |
69.4 |
229 |
79.3 |
206.3 |
73.9 |
229.1 |
73.7 |
NS, p=0.99 |
|
Altered triglycerides (%) * |
78.2 |
- |
83.1 |
- |
76.9 |
- |
86.9 |
- |
- |
Note. WG, Week of gestation at the time of GDM diagnosis;*Calculated with n = 398 where data were available; NS, non-significant; SD, standard deviation; a p-value of <0.05 was considered statistically significant; Mean and SD was calculated for continuous variables not for the percentages.
The influence of obesity on the diagnosis of GDM in our study was very clear: of the 153 patients diagnosed with GDM through the fasting glucose levels by the IADPSG criteria, 92% (141/153) had a BMI ≥ 25, which shows an increased risk of developing GDM when the patient is overweight, obese, or severely obese (OR 2.74, 95% CI 1.4-5.7). 16% of the patients with a normal BMI were diagnosed with GDM through the fasting glucose levels by the IADPSG criteria in comparison with the NICE criteria, which only detected 2.7 % (Figure 1A). At the final diagnosis, the percentage of patients classified with a normal BMI increased from 2.7% to 18% using the NICE criteria (Figure 1B). There was a direct relationship between fasting glucose levels and being overweight and obese. It was found that 80% of the patients with fasting glucose values below 92 mg/dL had a BMI> 25 while 100% of the patients with fasting glucose values greater than 99 mg/dL presented a BMI>25 (Figure 2A). 50% of the patients with values between 92-99 mg/dL presented insulin resistance by both methods (Figure 2B), which was present in 100% of the patients with GDM by both criteria when the value of glycemia was greater than 99 mg/dL (Figure 2C). In our cohort, the integrative value of both diagnostic criteria depended on the fasting glucose values of >92 mg/dL (n=153, 70%), plus the OGTT 1h determination (> 180mg / dl) (n = 27, 12.5%) from IADPSG; as well as the OGTT 2 h determination of >140 mg/dL from NICE (n=110, 60.5%), which were the three points of both criteria where it was possible to diagnose the majority of GDM patients in the Mexican population (Figure 3A).
Figure 1
Prevalence of GDM in patients with a normal BMI, overweight, obesity and severe obesity, both at fasting (A) and in the final diagnosis (B) using the IADPSG and NICE criteria.
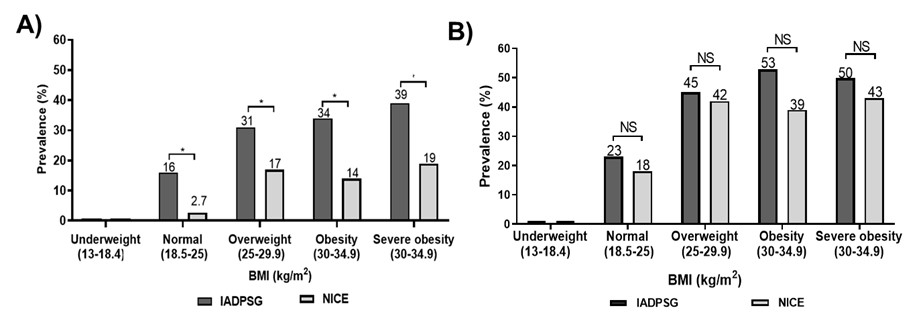
Note. Statistical test: paired t-Student’s test with a p-value of <0.05 and NS, non-significant.
Figure 2
Prevalence of BMI, GDM, and Insulin Resistance (IR) according to fasting glucose values.
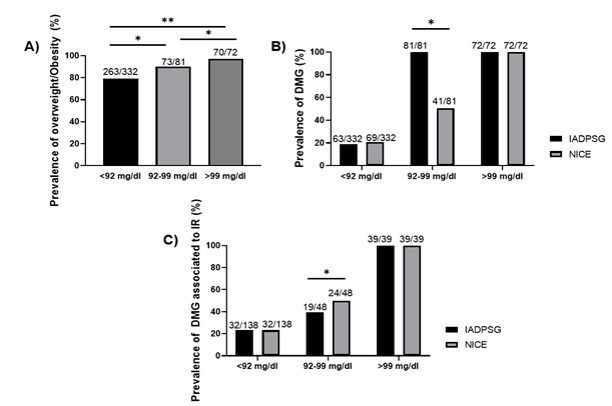
Note. Statistical test: Chi-square. A p-value of <0.05 was considered significant.
2.2. The use of only one criteria (IADPSG or NICE) limit the number of patients diagnosed with GDM
GDM patients diagnosed by IADPSG but misclassified as healthy by NICE were 12% of the cohort (n = 59); while those GDM patients diagnosed by NICE but misclassified by IADPSG as healthy were 5% (n=25). This meant that if only the NICE criteria were used, 2.5 women are not diagnosed with GDM, who would be diagnosed by IADPSG. Similarly, if only IADPSG were used, one woman would have not been diagnosed that in comparison, NICE would have detected (Figure 3B). Thus, using only the NICE criteria in our population leads to a greater loss of undiagnosed GDM cases. The characteristics of these groups of women (IADPSG positive, NICE negative) and (IADPSG negative, NICE positive) were quite similar (Table S2); nonetheless, the fasting glucose values and the number of women diagnosed in each time point of the OGTT showed the most interesting differences between groups. The diagnosis of GDM patients (IADPSG positive, NICE negative) (n=59) was performed for 68% of these patients with the fasting glucose value, showing an average value of 95.0 mg/dL. While the other 32% were diagnosed with the OGTT 1h, with an average glucose value 194 mg/dL. With the OGTT 2h, none of them was diagnosed. There has been a strong debate about the worth of OGTT 1h value; however, while analyzing this percentage of GDM patients and observing that just over a third part of this group is diagnosed with the OGTT 1h value make us confirm its importance for the diagnosis.
Figure 3
A) Percentage of GDM patients diagnosed with fasting glycemia, OGTT 1h and OGTT 2h for each criterion. B) Venn diagram showing the patients identified by both methods and those diagnosed that were mutually exclusive.
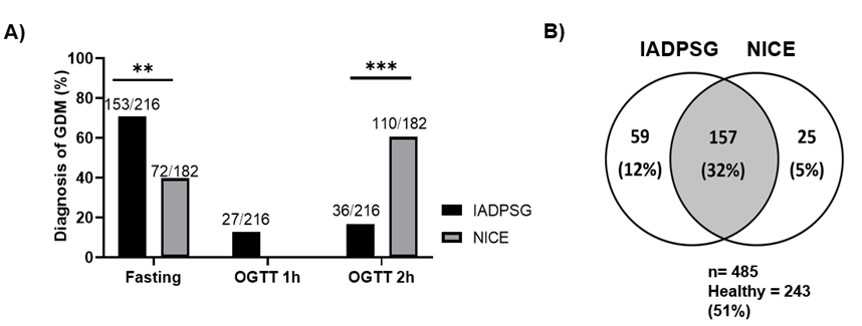
Note. Statistical test: Chi-square. A p-value <0.05 was considered significant.
2.3. Analysis of risk factors among diagnostic criteria in Mexican population
Among the risk factors, it was found that through IADPSG and NICE criteria, against healthy pregnant women, based on the fasting glucose levels, there were significant differences (p<0.05) in factors as age> 30, BMI> 25-30, BMI> 30, family history of T1DM/T2DM and obstetric problems (Table S3; Figure 4). It is worth to mention that in the fasting state, there was a strong association between the BMI>25 and a diagnosis of GDM for the NICE criteria. Among diagnostic criteria, there were no significant differences for the risk factors. In the comparative study of the risk factors and the GDM diagnosis, based on the glucose values of OGTT 2h, (Table S4; Figure S1) and in the final diagnosis (Table S5; Figure S2), the same trends described for GDM diagnosis based on fasting glucose levels were found (Figure S3). The fasting glucose levels showed significant difference (p<0.05) among GDM patients and healthy pregnant women in the insulin resistance and weight gain>20 kg when the IADPSG criteria were used, which was not seen with NICE criteria. In the OGTT 2h, this significant difference in the insulin resistance through IADPSG remains, which is not detected by NICE criteria. This implies that a group of GDM patients with insulin resistance were detected by IADPSG both with the fasting glucose levels and with the OGTT 2h, that is not identified by NICE, which shows that IADPSG criteria is stronger for the diagnosis of characteristics related to GDM and NICE seems to have more limitations.
Figure 4
Risk factors of patients diagnosed with GDM according to the fasting glucose levels by IADPSG and NICE criteria in a Mexican population.
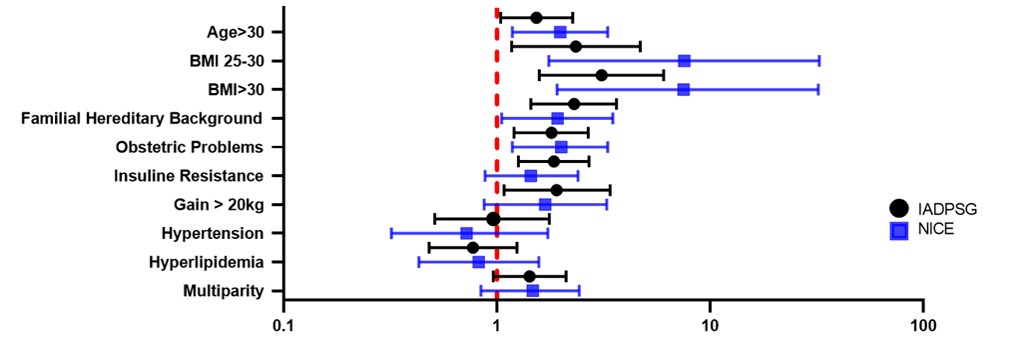
Note. Forest plot presenting the Odds Ratios (OR) and 95% confidence intervals.
Although the risk factors among groups seem to be the same, there are differences: in GDM patients, age>30 and insulin resistance are more increased; whereas, hyperlipidemia is elevated in healthy pregnant women compared to GDM patients. This indicates that, as age is older and insulin resistance higher, these women tend to have a positive GDM diagnosis when the IADPSG and NICE criteria are applied.
2.4. The analysis of outcomes did not show significant differences between GDM diagnosis by IADPSG or NICE criteria
The outcomes that were present more often both in GDM patients diagnosed by IADPSG and NICE are: induction of labor, cesarean section, respiratory distress syndrome, and requirement of oxygen. The outcomes that displayed a less frequency in both GDM groups were abortion, twin pregnancy and hypoglycemia. A remarkably high percentage of urinary tract infections (74%), cervicovaginitis (23.6%) or both (2%) occurred in patients with GDM. The maternal-fetal outcomes that showed a higher occurrence in patients with GDM according to IADPSG in comparison with healthy pregnant women were jaundice, respiratory distress syndrome, caesarean section, intubation, and oxygen requirement. While, according to NICE, they were hypoglycemia, jaundice, intubation and abortion. It is worth to mention that through NICE criteria, the association of hypoglycemia in GDM patients against healthy pregnant women is higher, as well as jaundice and requirement of intubation in both criteria. In general, IADPSG and NICE did not show significant differences in the presentation of outcomes (Tables S6 and S7; Figure 5).
Figure 5
Outcomes of patients diagnosed with GDM by IADPSG and NICE (A) and neonatal outcomes (B)
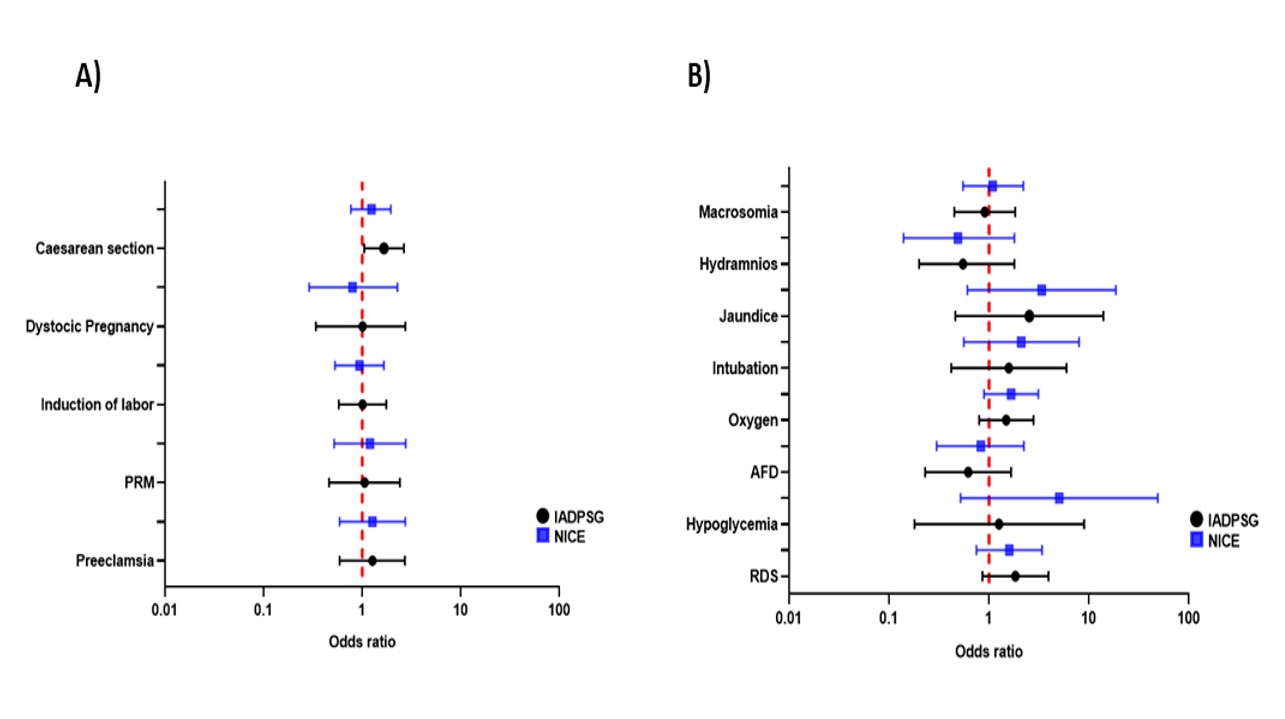
Note. Forest plot presenting the Odds Ratios (OR) and 95% confidence intervals. Acute Fetal Distress (AFD), Respiratory Distress Syndrome (RDS), Premature Rupture of Membranes (PRM). Only data comparable among both criteria has been included in the graphic presentation.
3. Discussion
In Mexico, it has been found that the prevalence of GDM oscillate between 8.7-17.7% (Ramírez-Torres, 2005; Hinojosa-Hernández et al., 2010; Forsbach-Sánchez et al., 2003). In general, there is still no consensus of a criterion for the diagnosis of GDM in the country (Font-López et al., 2017). In more recent studies, it was reported that by using the IADPSG criteria, the prevalence of GDM in Mexico is 30.1% (Reyes-Muñoz et al., 2012). The prevalence of GDM in the present study was 44.5% by IADPSG and 37.5% by NICE, one of the highest in the world. There are other countries with prevalence comparable, which have been determined with the IADPSG criteria, among them are: United Arab Emirates (45.3%) (Jenum et al., 2012), Spain (35.5%) (Duran et al., 2014), Saudi Arabia (41.5%) (Alfadhli, 2015), Bulgaria (31.6%) (Boyadzhieva et al., 2012) and India (41.9%) (Gopalakrishnan et al., 2015).
The BMI stratification of GDM patients in each criterion, allowed us to detect that the diagnosis of GDM in fasting state is affected by BMI in the IADPSG criteria showing a positive association: the higher the BMI, the greater the number of GDM cases diagnosed. This agrees with the findings that IADPSG seem to identify a profile more associated with obesity in comparison with NICE (Reichelt et al., 2017). Our results displayed that, for both criteria, the GDM groups presented a higher proportion of cesarean sections, macrosomia, requirement for oxygen, respiratory distress syndrome, and induction of labor compared to healthy pregnant women. For IADPSG, the outcomes that presented the higher association with GDM were jaundice, cesarean section and respiratory distress syndrome; while for the NICE, they were hypoglycemia, jaundice and of intubation. It is relevant to mention that for IADPSG, there was a significant difference between GDM and cesarean section in comparison with healthy pregnant women. By using NICE, it was found a greater association of abortions and hypoglycemia in GDM patients compared to healthy pregnant women. When comparing the outcomes of the GDM cohorts between criteria, no significant differences were found.
One of the most significant differences between IADPSG and NICE is the number of women with GDM diagnosis that are not detected if just one criterion is applied. We reiterate as necessary, the assessment of the IADPSG and NICE criteria to find the highest number of women at risk of GDM who can apply preventive/corrective measures. Other countries have proposed integrative comparisons as in Malaysia by comparing IADPSG and WHO criteria (Basri et al., 2018) and Korea who have made the same recommendation: use two diagnostic criteria, IADPSG and the Carpenter-Coustan (CC) criteria (Kim et al., 2019).
Studies that compare IADPSG and NICE criteria have reported that the outcomes tend to be more complicated with the IADPSG criteria, which is more robust than NICE for GDM diagnosis (Djelmis et al., 2016; Todi et al., 2020). This in agreement with a comparison among NICE and the ADA criteria, where the latter is more accurate in the detection of GDM patients and presents the least number of lost cases (Avalos et al., 2013). Also, it has been found that women classified by NICE as healthy tend to present complications that could be preventable (Reichelt et al., 2017) and take into account that results between criteria can differ even between regions per country (Kim et al., 2019). Comparative studies IADPSG vs NICE have been performed in Africa (Adam et al., 2017); Vietnam (Nguyen et al., 2020); England (Bhatia et al., 2018); Brazil (Reichelt et al., 2017) and Finland (Koivunen et al., 2020), all describe IADPSG as the criteria that detect more women with altered glucose levels during pregnancy. In the last three studies, except for some particular outcomes, both criteria presented similar characteristics and outcomes. Notably, it was reported that one of the diagnostic strengths of IADPSG versus NICE is the 1 hour after OGTT determination (Bhatia et al., 2018). In Latinoamerica (LA), comparative studies IADPSG vs NICE have been done in Argentina (Gorban de Lapertosa et al., 2020) and Brazil (Reichelt et al., 2017).
In line with these studies, when comparing the GDM cohorts (IADPSG versus NICE) the outcomes among both criteria did not show significant differences. This is related to the follow-up of the patients when they were diagnosed with GDM. The care program offered at the General Regional Hospital “Lic. Ignacio García Téllez” seeks the early and opportune diagnosis of GDM, personalized follow up and intensive treatment to achieve adequate metabolic control to significantly reduce maternal-fetal complications. Besides, monitoring of comorbidities associated with this pathology, support in the resolution of pregnancy, and tracing of metabolic status after delivery is performed. A characteristic that stands out in this population compared to other countries is the high rate of overweight and obese women that are diagnosed with GDM, therefore one of the approaches is focused on changing to a hypocaloric diet between 1500-1800 kilocalories per day depending on the BMI and without simple sugars, which was reviewed at each consultation. In this respect, among healthy patients there was an important percentage with elevated BMI and many of them presented different risk factors, which implies that Mexican population is at high-risk of development of GDM and/or other diseases, hence the importance of developing follow-up and detection programs for women with these characteristics.
There is a greater risk of developing GDM in Native American and Hispanic women than women without this ethnic origin (Brown et al., 2017). In Argentina, a GDM cohort diagnosed through NICE displayed very contrasting characteristics in comparison with the Mexican population: older mean age (34 years), lower levels of BMI 28 kg/m2 and glycated hemoglobin (5.1%) (Staltari et al., 2020). These show the profound population divergences among LA countries which difficult the application of a single GDM diagnostic criterion (in this case, NICE), as has been proposed by ALAD for all LA. The implementation of both criteria for the diagnosis of GDM at a national level is a challenge. However, we have shown the strength of the IADPSG against NICE criteria: it detects a greater number of patients at risk from fasting glycemic values between 92-99 mg/dL, finds greater number of GDM patients with insulin resistance, the OGTT 1h value allows to find a percentage of patients that NICE criteria miss because of the lack of this time point determination and thus, there are fewer cases of GDM lost as “healthy”. The joint use of NICE criteria would allow the detection of a lower percentage of GDM cases classified as healthy by IADPSG, which would be complementary in the GDM diagnosis.
The strengths of this study include an analysis of pregnant patients without biasing them given their risk antecedents. The patients belonged to the northeast region of Mexico, where this report is unique in its kind. Furthermore, this is one of the first studies performed in LA that compare both criteria. One of the strongest observations of this study implies that given the high prevalence of risk factors for GDM in Mexican women, a universal screening for GDM should be considered in all pregnant women in our country. Among the limitations of the study, it does not fully reflect the entire Mexican population where the divergence in the ethnic component is significant. In addition, the studied groups could have greater statistical power if their numbers increased. Future research that expands these explorations will contribute to the study of GDM in Mexico.
4. Conclusions
The clinical observations from this study imply the necessity of exploring both criteria (IADPSG versus NICE) for GDM diagnosis, which can vary among countries in LA. The adoption of both criteria for GDM diagnosis in Mexico is focused on the detection of the largest number of pregnant women with altered glycemic values. The intrinsic characteristics of the cohort, as well as its risk factors, allowed us to understand the high susceptibility of the Mexican population to suffer obesity, which directly influences the possibility of developing GDM. Finally, the exploration of the outcomes and the lack of significant differences between criteria highlight the importance of the follow-up programs for GDM women, which is currently under exploration in our population to implement actionable preventive/corrective measures aimed to avoid adverse maternal-fetal outcomes.
Acknowledgments
We are thankful to Chávez, R., Oviedo, J.C., Gutiérrez-Sánchez, J.M., Tabet-Sandoval, M.A., Reta-García, R.J., Lozano-Guzmán, I., Pancardo-Amador, M.J., Múñiz-Reséndiz, V.R., Santos-Cruz, del Á. and Martínez-Álvarez, G. for their contribution in data collection or/and management.
Statement of data availability
Data used in this research is available upon request
References
Adam, S. y Rheeder, P. (2017). Screening for gestational diabetes mellitus in a South African population: Prevalence, comparison of diagnostic criteria and the role of risk factors. South African Medical Journal, 107(6), 523–527. https://doi.org/10.7196/SAMJ.2017.v107i6.12043
Alfadhli, E. (2015). Gestational diabetes in Saudi women identified by the International Association of Diabetes and Pregnancy Study Group versus the former American Diabetes Association criteria: a prospective cohort study. Annals of Saudi Medicine, 35 (6), 428–434. https://doi.org/10.5144/0256-4947.2015.428
Avalos, G.E., Owens, L.A., Dunne, F. y ATLANTIC DIP Collaborators. (2013) Applying current screening tools for gestational diabetes mellitus to a European population: is it time for change? Diabetes Care, 36(10), 3040–3044. https://doi.org/10.2337/dc12-2669
Basri, N.I., Mahdy, Z.A., Ahmad, S., Karim, A.K.A., Shan, L.P., Manaf, M.R.A. y Ismail, N.A.M. (2018). The World Health Organization (WHO) versus The International Association of Diabetes and Pregnancy Study Group (IADPSG) diagnostic criteria of gestational diabetes mellitus (GDM) and their associated maternal and neonatal outcomes. Hormone Molecular Biology and Clinical Investigation, 34(1). https://doi.org/10.1515/hmbci-2017-0077
Bhatia, M., Mackillop, L.H., Bartlett, K., Loerup, L., Kenworthy, Y., Levy, J.C.… y Hirst, J.E. (2018) Clinical Implications of the NICE 2015 Criteria for Gestational Diabetes Mellitus. Journal of Clinical Medicine, 7(10), 376. https://doi.org/10.3390/jcm7100376
Billionnet, C., Mitanchez, D., Weill, A., Nizard, J., Alla, F., Hartemann, A.… y Jacqueminet, S. (2017). Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia, 60, 636-644. https://doi.org/10.1007/s00125-017-4206-6
Boyadzhieva, M.V., Atanasova, I., Zacharieva, S., Tankova, T. y Dimitrova, V. (2012). Comparative analysis of current diagnostic criteria for gestational diabetes mellitus. Obstetric Medicine, 5(2), 71–77. https://doi.org/10.1258/om.2011.110073
Brown, F.M. y Wyckoff, J. (2017) Application of One-Step IADPSG Versus Two-Step Diagnostic Criteria for Gestational Diabetes in the Real World: Impact on Health Services, Clinical Care, and Outcomes. Current Diabetes Reports, 17(10), 85. doi: https://doi.org/10.1007/s11892-017-0922-z
Djelmis, J., Pavic, M., Kotori, V.M., Renar, I.P., Ivanisevic, M. y Oreskovic, S. (2016) Prevalence of gestational diabetes mellitus according to IADPSG and NICE criteria. International Journal of Gynaecology and Obstetrics, 135(3), 250–254. https://doi.org/10.1016/j.ijgo.2016.07.005
Duran, A., Sáenz, S., Torrejón, M.J., Bordiú, E., Del Valle, L., Galindo, M., …y Calle-Pascual A.L. (2014). Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care, 37(9), 2442–2250. https://doi.org/10.2337/dc14-0179
Font-López, K.C. y Gutiérrez-Castañeda, M.R. (2017). Diagnóstico de diabetes gestacional en población mexicana. Ginecología y Obstetricia de México, 85(2), 116–124.
Forsbach-Sánchez, G., González-Obele, E., Villanueva-Cuellar, M.A., Tamez-Pérez, H.E., y Rocha-Márquez, J. (2003). Impacto del nuevo criterio para el diagnóstico de diabetes gestacional en la estimación de su prevalencia. Revista de Investigación Clínica, 55(5), 507–510.
Gopalakrishnan, V., Singh, R., Pradeep, Y., Kapoor, D., Rani, A.K., Pradhan S. … y Yadav, S. B. (2015). Evaluation of the prevalence of gestational diabetes mellitus in North Indians using the International Association of Diabetes and Pregnancy Study groups (IADPSG) criteria. Journal of Postgraduate Medicine, 61(3), 155–158. https://doi.org/10.4103/0022-3859.159306
Gorban de Lapertosa, S., Sucanis, S., Salzberg, S., Alvariñas, J., Faingold, C., Jawerbaum, A. … y DSPG-SAD Group. (2020). Prevalence of gestational diabetes mellitus in Argentina according to the Latin American Diabetes Association (ALAD) and International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria and the associated maternal-neonatal complications. Health Care Women International, 42(4-6), 636-656. https://doi.org/10.1080/07399332.2020.1800012
HAPO Study Cooperative Research Group, Metzger, B.E., Lowe, L.P., Dyer, A.R., Trimble, E.R., Chaovarindr, U., … y Sacks, D.A. (2008). Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine, 358 (19), 1991–2002. https://doi.org/10.1056/NEJMoa0707943
Hinojosa-Hernández, M.A., Hernández-Aldana, J.H., Barrera-Tenorio, E.F., Gayosso y Martínez, M.T. (2010). Prevalencia de diabetes mellitus gestacional en el Hospital Juárez de México. Revista del Hospital Juárez de México, 77(2), 123–128.
International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger, B., Gabbe, S. G., Persson, B., Buchanan, T. A., Catalano, P.A. ... y Schmidt, M. I. (2010). International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care, 33(3), 676–682. https://doi.org/10.2337/dc09-1848
Jenum, A.K., Mørkrid, K., Sletner, L., Vangern, S., Torper, J.L., Nakstad, B., … y Birkeland, K. I. (2012). Impact of ethnicity on gestational diabetes identified with the WHO and the modified International Association of Diabetes and Pregnancy Study Groups criteria: a population-based cohort study. European Journal of Endocrinology, 166(2), 317–324. https://doi.org/10.1530/EJE-11-0866
Johns, E.C., Denison, F.C., Norman, J.E., Reynolds, R.M. (2018). Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends in Endocrinology and Metabolism, 29(11), 743-754. https://doi.org/10.1016/j.tem.2018.09.004
Kamana, K.C,, K., Shakya, S., y Zhang, H. (2015). Gestational diabetes mellitus and macrosomia: a literature review. Annals of Nutrition and Metabolism, 66(Suppl 2), 14–20. https://doi.org/10.1159/000371628
Kautzky-Willer, A., Harreiter, J., Winhofer-Stöckl, Y., Bancher-Todesca, D., Berger, A., Repa, A., ... y Weitgasser, R. (2019). [Gestational diabetes mellitus (Update 2019)]. Wiener Klinsiche Wochenschrift, 131 (Suppl 1), 91–102. https://doi.org/10.1007/s00508-018-1419-8
Kim, S.H, England, L., Wilson, H.G., Bish, C., Satten, G.A., Dietz, P. (2010). Percentage of gestational diabetes mellitus attributable to overweight and obesity. American Journal of Public Health, 100(6), 1047-52. https://doi.org/10.2105/AJPH.2009.172890
Kim, M.H., Kwak, S.H., Kim, S.H., Hong, J.S., Chung, H.R., Choi, S.H., … y Jang, H. C. (2019). Pregnancy Outcomes of Women Additionally Diagnosed as Gestational Diabetes by the International Association of the Diabetes and Pregnancy Study Groups Criteria. Diabetes and Metabolism Journal, 43(6), 766–775. https://doi.org/10.4093/dmj.2018.0192
Koivunen, S., Viljakainen, M., Männistö, T., Gissler, M., Pouta, A., Kaaja, R., … y Vääräsmäki, M. (2020). Pregnancy outcomes according to the definition of gestational diabetes. PLoS One, 15 (3), e0229496. https://doi.org/10.1371/journal.pone.0229496
Nguyen, C.L., Lee, A.H., Pham, N.M., Nguyen, P.T.H., Van Ha, A.V., Chu, T.K … y Binns, C. W. (2020). Prevalence and pregnancy outcomes of gestational diabetes mellitus by different international diagnostic criteria: a prospective cohort study in Vietnam. Journal of Maternal-Fetal and Neonatal Medicine, 33(21), 3706–3712. https://doi.org/10.1080/14767058.2019.1583733
Ramírez-Torres, M.A. (2005). Diabetes mellitus gestacional. Experiencia en una institución de tercer nivel de atención. Ginecología y Obstetricia de México, 73(9), 484–491.
Reichelt, A.J., Schwerz-Weinert, L., Silveira-Mastella, L., Gnielka, V., Campos, M.A., Hirakata, V.N.,…y Schmidt, M. I. (2017). Clinical characteristics of women with gestational diabetes - comparison of two cohorts enrolled 20 years apart in southern Brazil. Sao Paulo Medical Journal, 135(4), 376–382. https://doi.org/10.1590/1516-3180.2016.0332190317
Reyes-Muñoz, E., Parra, A., Castillo-Mora, A. y Ortega-Gonzalez, C. (2012). Effect of the diagnostic criteria of the International Association of Diabetes and Pregnancy Study Groups on the prevalence of gestational diabetes mellitus in urban Mexican women: a cross-sectional study. Endocrine Practice, 18(2), 146–151. https://doi.org/10.4158/EP11167.OR
Salzberg, S., Alvariñas, J., López, G., Gorbán de Lapertosa, S., Linari, M.A., Falcón, E., …y Barba, A.M. (2016). Guías de diagnóstico y tratamiento de diabetes gestacional. Revista de la Asociación Latinoamericana de Diabetes, 6, 155–169. http://dx.doi.org/10.47196/diab.v50i3.45
Staltari, B.J., Cutó, F.G., Merlo, C., Garcés, N., Benkovic, R.I., de Loredo, S., y de Loredo, L. (2020). Prevalence of gestational diabetes, reclassification and results after delivery in patients attended at the University Private Hospital of Cordoba. Revista de la Asociación Latinoamericana de Diabetes, 10, 47–53.
Todi, S., Sagili, H., y Kamalanathan, S.K. (2020) Comparison of criteria of International Association of Diabetes and Pregnancy Study Groups (IADPSG) with National Institute for Health and Care Excellence (NICE) for diagnosis of gestational diabetes mellitus. Archives of Gynecology and Obstetrics, 302(9), 47–52. https://doi.org/10.1007/s00404-020-05564-9
Torloni, M.R., Betrán, A.P., Horta, B.L., Nakamura, M. U., Atallah, A. N., Moron, A.F., y Valente, O. (2009). Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obesity Reviews, 10(2), 194-203. https://doi.org/10.1111/j.1467-789X.2008.005
Appendix A. Supplementary tables and figures
Table S1
Weight comparison by gender of newborns from GDM patients diagnosed by IADPSG and NICE criteria in a Mexican population
|
Characteristics
|
Male |
Female |
||||||||
|
IADPSGa |
NICEb |
Difference among criteria |
IADPSGa |
NICEb |
Difference among criteria |
|||||
|
|
Mean |
SD |
Mean |
SD |
|
Mean |
SD |
Mean |
SD |
|
|
n |
111 |
- |
96 |
- |
- |
94 |
- |
80 |
- |
- |
|
%* |
54.1 |
- |
54.5 |
- |
- |
45.9 |
- |
45.5 |
- |
- |
|
Weight |
3.2 |
0.5 |
3.3 |
0.5 |
NS, p=0.8 |
3.3 |
0.6 |
3.2 |
0.6 |
NS, p=0.6 |
|
Height |
50.2 |
3.2 |
50.2 |
3.3 |
NS, p=0.99 |
49.8 |
2.5 |
49.7 |
2.8 |
NS, p=0.9 |
|
WGB |
38.1 |
1.3 |
38.1 |
1.3 |
NS, p=0.9 |
37.9 |
1.3 |
37.8 |
1.4 |
NS, p=0.9 |
Note. Week of gestation at the time of birth (WGB); * Percentage of presentation; a. Available data, n = 205; b. Available data, n = 176; NS, non-significant; SD, standard deviation; a p-value of <0.05 was considered statistically significant. Mean and SD was calculated for continuous variables not for the percentages.
Table S2
Characteristics of patients with GDM diagnosed only by IADPSG or NICE criteria (mutually exclusive) in a Mexican population.
|
Characteristics |
IADPSG |
NICE |
||
|
Mean |
SD |
Mean |
SD |
|
|
n |
59 |
- |
25 |
- |
|
Age (years) |
31.1 |
4.6 |
31.3 |
6.5 |
|
BMI |
32.5 |
6.4 |
31.4 |
5.5 |
|
Normal weight (%) |
11.9 |
- |
12 |
- |
|
Overweight (%) |
88.1 |
- |
88 |
- |
|
Obesity (%) |
0 |
- |
0 |
- |
|
WG |
26 |
6.5 |
26.6 |
6.7 |
|
Glycosylated hemoglobin (%) |
5.5 |
0.5 |
5.4 |
0.4 |
|
Cholesterol (mg/dl) * |
217 |
42.3 |
233.4 |
54.1 |
|
Altered Cholesterol (%) * |
64.7 |
- |
76.2 |
- |
|
Triglycerides (mg/dl) * |
224.7 |
84.9 |
219.4 |
54.4 |
|
Altered triglycerides (%) * |
78.4 |
- |
95.2 |
- |
Note. SD, standard deviation; Week of gestation at the time of GDM diagnosis (WG). Mean and SD was calculated for continuous variables not for the percentages.
Table S3
Risk factors of patients diagnosed with GDM in fasting state by IADPSG and NICE criteria in a Mexican population
|
Risk Factor |
IADPSG |
NICE |
||||||
|
|
Percentage * |
p-value |
OR |
CI:95% |
Percentage * |
p-value |
OR |
CI:95% |
|
Age>30 |
55.6 |
0.029 |
1.53 |
1.04-2.26 |
62.5 |
0.009 |
1.98 |
1.18-3.30 |
|
BMI 25-30 |
35.3 |
0.015 |
2.34 |
1.17-4.69 |
41.7 |
0.0017 |
7.55 |
1.75-32.48 |
|
BMI >30 |
56.9 |
0.0007 |
3.09 |
1.58-6.04 |
55.6 |
0.0016 |
7.5 |
1.91-32.12 |
|
Hereditary background |
81.0 |
0.0003 |
2.30 |
1.44-3.63 |
80.6 |
0.036 |
1.92 |
1.05-3.49 |
|
Obstetric problems |
41.2 |
0.004 |
1.80 |
1.20-2.68 |
45.8 |
0.007 |
2.00 |
1.18-3.30 |
|
Insulin resistance |
56.9 |
0.0020 |
1.85 |
1.26-2.70 |
54.2 |
NS, 0.16 |
1.44 |
0.88-2.40 |
|
Gain > 20kg |
16.3 |
0.025 |
1.90 |
1.08-3.39 |
16.7 |
NS, 0.14 |
1.68 |
0.87-3.27 |
|
Hypertension |
10.5 |
NS, 0.90 |
0.96 |
0.51-1.76 |
8.3 |
NS, 0.48 |
0.72 |
0.32-1.73 |
|
Hyperlipidemia |
79.1 |
NS, 0.28 |
0.77 |
0.48-1.24 |
79.2 |
NS, 0.52 |
0.82 |
0.43-1.57 |
|
Multiparity |
42.8 |
NS, 0.083 |
1.42 |
0.96-2.11 |
45.1 |
NS, 0.13 |
1.47 |
0.87-2.43 |
Note. * Percentage of presentation (Calculated only in patients with GDM); NS Non-significant; OR, Odds Ratio; CI, confidence interval. A p-value of <0.05 was considered statistically significant.
Table S4
Risk factors of patients diagnosed with GDM at OGTT 2h by IADPSG and NICE criteria in a Mexican population
|
Risk Factor |
IADPSG |
NICE |
|||||||
|
|
Percentage * |
p-value |
OR |
CI:95% |
Percentage * |
p-value |
OR |
CI:95% |
|
|
Age>30 |
60.0 |
0.0039 |
1.86 |
1.21-2.85 |
60 |
0.00014 |
2.08 |
1.42-3.04 |
|
|
BMI 25-30 |
38.3 |
0.0376 |
2.18 |
1.03-4.43 |
39.4 |
0.0011 |
2.95 |
1.51-5.79 |
|
|
BMI >30 |
53.0 |
0.0235 |
2.27 |
1.10-4.73 |
52.9 |
0.0008 |
2.96 |
1.57-5.75 |
|
|
Hereditary background |
81.7 |
0.002 |
2.25 |
1.36-3.80 |
80.6 |
0.00021 |
2.29 |
1.48-3.58 |
|
|
Obstetric problems |
40.9 |
0.022 |
1.66 |
1.08-2.56 |
41.2 |
0.002 |
1.86 |
1.27-2.73 |
|
|
Insulin resistance |
59.1 |
0.0020 |
1.96 |
1.29-2.98 |
51.8 |
NS, 0.081 |
1.39 |
0.95-2.01 |
|
|
Gain > 20kg |
13.9 |
NS, 0.36 |
1.33 |
0.70-2.44 |
12.4 |
NS, 0.68 |
1.13 |
0.64-2.02 |
|
|
Hypertension |
8.7 |
NS, 0.42 |
0.74 |
0.37-1.49 |
10.0 |
NS, 0.70 |
0.89 |
0.49-1.61 |
|
|
Hyperlipidemia |
76.5 |
NS, 0.089 |
0.64 |
0.38-1.07 |
78.2 |
NS, 0.13 |
0.70 |
0.43-1.12 |
|
|
Multiparity |
41.6 |
NS, 0.26 |
1.28 |
0.84-1.96 |
45.2 |
0.007 |
1.69 |
1.15-2.49 |
|
Note. * Percentage of presentation (Calculated only in patients with GDM); NS Non-significant; OR, Odds Ratio; CI, confidence interval. A p-value of <0.05 was considered statistically significant.
Table S5
Risk factors of patients diagnosed with GDM by final diagnosis by IADPSG and NICE criteria in a Mexican population
|
|
IADPSG |
NICE |
||||||||
|
Risk Factor |
Healthy |
GDM |
Healthy |
GDM |
||||||
|
|
Percentage* |
Percentage§ |
p-value |
OR |
CI:95% |
Percentage╚ |
Percentage¤ |
p-value |
OR |
CI:95% |
|
Age>30 |
39.8 |
58.8 |
0.000031 |
2.16 |
1.50-3.12 |
41.9 |
58.8 |
0.00032 |
1.98 |
1.36-2.86 |
|
BMI 25-30 |
35.7 |
36.1 |
0.0011 |
2.74 |
1.51-5.05 |
33.3 |
40.1 |
0.0002 |
3.41 |
1.75-6.81 |
|
BMI >30 |
41.6 |
56 |
0.0011 |
3.62 |
2.00-6.44 |
45.2 |
52.7 |
0.0002 |
3.29 |
1.75-6.38 |
|
Hereditary background |
64.3 |
77.3 |
0.002 |
1.89 |
1.25-2.82 |
64.0 |
80.2 |
0.00017 |
2.28 |
1.47-3.52 |
|
Obstetric problems |
27.1 |
38.4 |
0.008 |
1.67 |
1.14-2.47 |
27.4 |
40.1 |
0.0036 |
1.78 |
1.20-2.64 |
|
Insulin resistance |
39.4 |
55.1 |
0.0006 |
1.89 |
1.32-2.71 |
42.9 |
52.2 |
0.047 |
1.45 |
1.00-2.12 |
|
Gain > 20kg |
9.7 |
13.9 |
NS, 0.15 |
1.5 |
0.85-2.60 |
10.9 |
12.6 |
NS, 0.56 |
1.19 |
0.66-2.10 |
|
Hypertension |
10.8 |
10.6 |
NS, 0.96 |
0.99 |
0.54-1.73 |
11.2 |
9.9 |
NS, 0.65 |
0.87 |
0.48-1.56 |
|
Hyperlipidemia |
83.6 |
79.6 |
NS, 0.25 |
0.77 |
0.49-1.20 |
83.8 |
78.6 |
NS, 0.15 |
0.7 |
0.44-1.13 |
|
Multiparity |
32.3 |
43 |
0.017 |
1.57 |
1.08-2.26 |
33.0 |
43.9 |
0.018 |
1.58 |
1.09-2.3 |
Note. Healthy patients according to IADPSG criteria *(n=269); GDM patients according to IADPSG criteria§ (n=216). Healthy patients according to NICE criteria ╚(n=303); GDM patients according to NICE criteria ¤(n=182); OR, Odds Ratio; CI, confidence interval. A p-value of <0.05 was considered statistically significant.
Table S6
Outcomes of patients diagnosed with GDM by IADPSG and NICE criteria in a Mexican population
|
|
IADPSG |
NICE |
||||||||
|
Outcome |
Healthy |
GDM |
Healthy |
GDM |
||||||
|
|
Percentage |
Percentage |
p-value |
OR |
CI:95% |
Percentage |
Percentage |
p-value |
OR |
CI:95% |
|
Preeclampsia |
5.2* |
6.5§ |
NS, 0.55 |
1.27 |
0.59-2.71 |
5.3╚ |
6.6¤ |
NS, 0.55 |
1.27 |
0.59-2.74 |
|
Urinary tract infection |
81.0* |
74.5§ |
- |
- |
- |
78.9╚ |
76.9¤ |
- |
- |
- |
|
Cervicovaginitis |
18.2* |
23.6§ |
- |
- |
- |
20.1╚ |
21.4¤ |
- |
- |
- |
|
Both# |
0.7* |
1.9§ |
- |
- |
- |
1.0╚ |
1.6¤ |
- |
- |
- |
|
Premature rupture of membranes |
4.8* |
5.1§ |
NS, 0.9 |
1.06 |
0.46-2.41 |
4.6╚ |
5.5¤ |
NS, 0.67 |
1.2 |
0.52-2.76 |
|
Labor induction |
11.9* |
12§ |
NS, 0.96 |
1.01 |
0.58-1.76 |
12.2╚ |
11.5¤ |
NS, 0.83 |
0.94 |
0.53-1.66 |
|
Abortion |
0* |
0.9§ |
NS, 0.2 |
- |
- |
0.3╚ |
0.5¤ |
NS, 0.99 |
1.67 |
0.10-26.84 |
|
Distocic pregnancy |
4.1* |
2.8§ |
NS, 0.98 |
1.01 |
0.34-2.74 |
4.0a |
2.7¤ |
NS, 0.71 |
0.8 |
0.29-2.28 |
|
Cesarean section |
72.5* |
81.0§ |
0.038 |
1.66 |
1.05-2.65 |
75.1a |
79.1¤ |
NS, 0.38 |
1.24 |
0.77-1.95 |
|
Twin pregnancy |
1.1* |
0.9┼ |
NS, 0.99 |
1.26 |
0.20-8.10 |
1.3a |
0^ |
NS, 0.30 |
- |
- |
Note. # Urinary tract infection and cervicovaginitis; Healthy patients according to IADPSG criteria *(n=269) and ¶(n=268); GDM patients according to IADPSG criteria § (n=216) and ┼(n=214). Healthy patients according to NICE criteria ╚(n=303) and a(n=301); GDM patients according to NICE criteria ¤(n=182) and ^ (n=181). NS Non-significant; OR, Odds Ratio; CI, confidence interval; -, Does not apply. A p-value of <0.05 was considered statistically significant.
Table S7
Neonatal outcomes from patients diagnosed with GDM by IADPSG and NICE criteria in a Mexican population
|
|
IADPSG |
NICE |
||||||||
|
Outcome |
Healthy |
GDM |
Healthy |
GDM |
||||||
|
|
Percentage |
Percentage |
p-value |
OR |
CI:95% |
Percentage |
Percentage |
p-value |
OR |
CI:95% |
|
Hypoglycemia |
0.7¶ |
0.9┼ |
NS, 0.99 |
1.26 |
0.18-8.98 |
0.3a |
1.7^ |
NS, 0.15 |
5.06 |
0.52-48.97 |
|
Respiratory distress syndrome |
4.5¶ |
7.9┼ |
NS, 0.11 |
1.84 |
0.86-3.94 |
5.0a |
7.7^ |
NS, 0.22 |
1.6 |
0.75-3.40 |
|
Acute fetal distress |
4.5¶ |
2.8┼ |
NS, 0.34 |
0.62 |
0.23-1.67 |
4.0a |
3.3^ |
NS, 0.7 |
0.83 |
0.30-2.24 |
|
Oxygen requirement |
7.5¶ |
10.7┼ |
NS, 0.21 |
1.49 |
0.80-2.80 |
7.3a |
11.6^ |
NS, 0.11 |
1.67 |
0.89-3.12 |
|
Intubation requirement |
1.5¶ |
2.3┼ |
NS, 0.52 |
1.58 |
0.42-5.96 |
1.3a |
2.8^ |
NS, 0.31 |
2.11 |
0.56-7.96 |
|
Jaundice |
0.7¶ |
1.9┼ |
NS, 0.41 |
2.53 |
0.46-13.97 |
0.7 a |
2.2^ |
NS, 0.20 |
3.38 |
0.61-18.63 |
|
Hydramnios |
3.4¶ |
1.9┼ |
NS, 0.32 |
0.55 |
0.20-1.80 |
3.3 a |
1.7^ |
NS, 0.39 |
0.49 |
0.14-1.80 |
|
Macrosomia |
8.2¶ |
7.5┼ |
NS, 0.8 |
0.91 |
0.45-1.83 |
7.6 a |
8.3^ |
NS, 0.8 |
1.09 |
0.55-2.21 |
Note. Healthy patients according to IADPSG criteria *(n=269) and ¶(n=268); GDM patients according to IADPSG criteria § (n=216) and ┼(n=214). Healthy patients according to NICE criteria ╚(n=303) and a(n=301); GDM patients according to NICE criteria ¤(n=182) and ^ (n=181). NS Non-significant; OR, Odds Ratio; CI, confidence interval; -, Does not apply. A p-value of <0.05 was considered statistically significant.
Figure S1
Risk factors of patients diagnosed with GDM by OGTT 2 hours with IADPSG and NICE criteria in a Mexican population. Forest plot presenting Odds ratios (OR) and 95% confidence intervals.
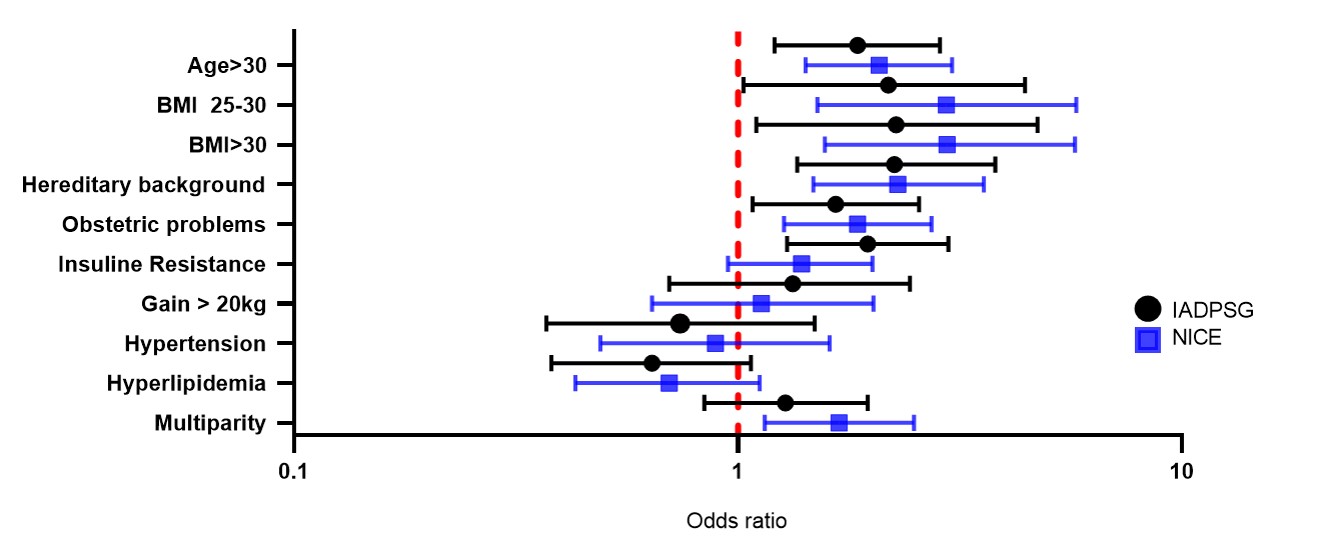
Figure S2
Risk factors of patients diagnosed with GDM by final diagnosis with IADPSG and NICE criteria in a Mexican population. Forest plot presenting Odds ratios (OR) and 95% confidence intervals.
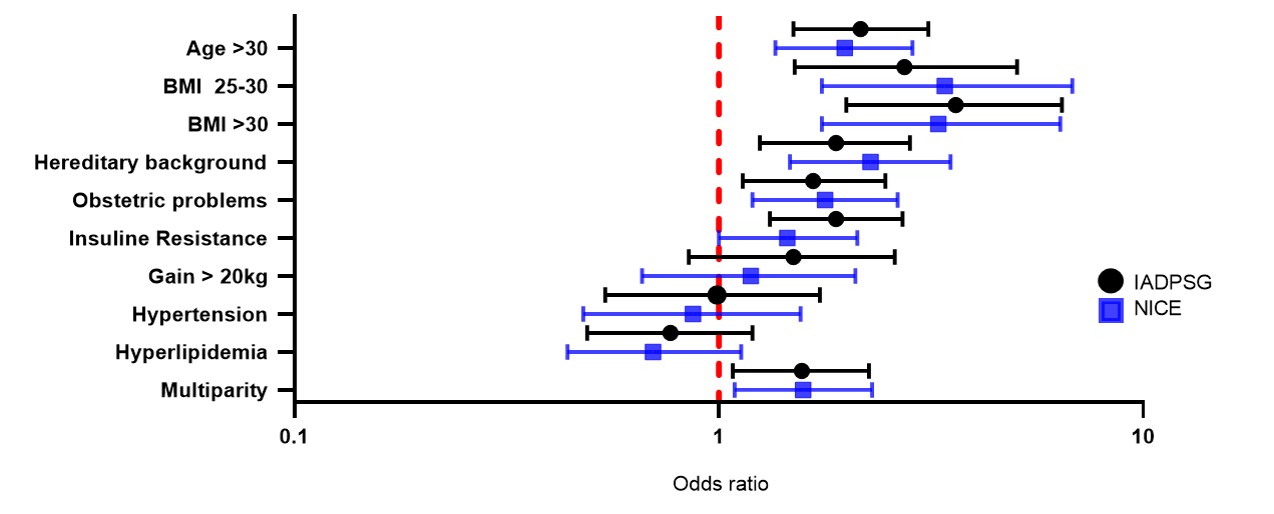
Figure S3
Risk factors associated with the prevalence of GDM by final diagnosis using the IADPSG and NICE criteria