Life and Earth Environment from an Entropic Point of View
Masaki Hayashi
School of Life Science, Tokyo University of Pharmacy and Life Science
E-mail: hayashi@ls.toyaku.ac.jpand
ABSTRACT
At a first glance it seems that the Second Law of Thermodynamics, the Fundamental Law of Physics, is inapplicable to a system of life and to the
environment of Earth. It is understandable, however, if one takes into account that both systems represent themselves non–equilibrium stationary open
systems. A deeper understanding to these systems becomes possible if one considers them from an entropic approach. Such considerations could provide
interesting educational materials for the beginners of thermodynamics. Key Word: Thermodynamics, physics, entropy, living organism.
RESUMEN
A primera vista La Segunda Ley de la Termodinámica, la Segunda Ley de la Física, parece no ser aplicable a un sistema de vida ni al medio ambiente de
la Tierra. Esto se debe a que La Segunda Ley de la Termodinámica esta formulada en un marco de trabajo de un sistema de equilibrio cerrado, mientras
que ambos representan, por sí mismos, sistemas estacionarios abiertos no equilibrados. Usualmente éstos no se tratan en el curso introductorio de
termodinámica. Un entendimiento profundo de estos sis- temas es posible si los consideramos desde el punto de vista entrópico. Tales consideraciones
deben proveer interesante materia educativa para principiantes de la termodinámica.
Palabras Claves: Termodinámica, física, entropía, organismo vivo.
1.LIFE AND ENTROPY
The structure order of a living organism might appear rather mysterious from a naive point of view in physics. Its entropy does not increase and never
comes to a thermal equilibrium state, i.e., to a disorder. Actually, the entropy is kept at a low level and apparently does not increase in various
biological phenomena such as mainte- nance and growth of an individual living body, as well as a self–reproduction. In contrast, after death the
decomposition of a body starts to diffuse as usual substances do and hence the entropy increases, approaching to a disordered state. Thus it seems as
if the second law of ther- modynamics were inapplicable to a living orga-
nism [1,2]. The difficulty arising from this physics viewpoint cannot be saved by a biologi- cal explanation such as metabolism and/or assimilation of
plants. E. Schrödinger gave the following explanation [1]:
1.1 Explanation by Schrödinger
Schrödinger argued in his book "What is life?" as "A living organism takes negative entropy (negentropy)", and emphasized the importance of
consideration based on an entropy concept. Since a living organism is in a stationary state, the flow of outgoing energy E out must
be equal
to that of the incoming energy E in. Thus on average one has
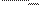
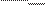 dE
in
=dE
out
dE
in
=dE
out
dt
dt
(Ec. 1)
The incoming energy carries the entropy with it as well. He paid attention to the difference of the incoming entropy Sin and the
outgoing one Sout and noticed that:
DS =Sin - Sout
(Ec. 2)
A living organism is in a non-equilibrium state, and because irreversible processes take place therein, entropy should be generated. Conse- quently the
net entropy accepted by a living organism has to be negative. Thus a living orga- nism maintains its living state by taking "negen- tropy", i.e., by
compensating the entropy gene- rated through the irreversible processes.
After all, the living organism throws the entropy to the surrounding and thereby increa- ses the entropy of the surrounding. As a result it preserves
its entropy at a lower level hence its oredered (molecular) structures in an ordered state.
1.2 Entropic Income and Expenditure in a Living Organism.
A living organism constitutes an open system that exchanges heat and substances with the environment. Moreover it is a stationary system that does not
change much in a long period of time. In order that a living organism can main- tain its stationarity, it has to discharge outside the same amount of
the substances and the energy taken in from outside. They become excretions and abolition heat, respectively, which go out to the environment. Since
entropy is newly generated due to the irreversible processes within the system, a living organism has to throw a necessary amount for compensa- tion.
As a result, the entropy in the system is preserved at a lower level.
1.3 What is the Mechanism by Which Entropy is Thrown Away?
There are two kinds of ways. One way is thro- wing entropy by attaching it to waste and the other way is attaching it to abolition heat. One cannot
throw entropy alone without attaching to anything. A living organism burns, for instance, the carbohydrate in the body and transforms substances such
as carbon dioxide and steam into high entropy, and exhausts them outside of the body mainly by breathing. Thus the entropy generated by the activity of
a living organism is partly thrown away outside of the body as high entropy waste. The entropy can be thrown away as well in the form of the thermal
energy transfer such as the evaporations and/or sweat. In this way a living organism maintains its stationarity.
After all, what are useful for a living organism are those substances possessing not only high energetic states but also low entropic states. In order
that a living organism can function, it is necessary not only to take such low entro- py substances but also to discharge high entropy substances and
heat into the environ- ment. For this purpose it is necessary conside- ring a mechanism of entropy abolition from the environment surrounding living
organisms.
1.4 Low Entropic Sunlight
The sunlight energy plays a crucial role in main- taining life. The fact that it possess a low level of entropy is physically more important than an
energetic aspect. Let us consider here a physi- cal mechanism of photosynthesis in a plant. The photosynthesis of a plant is the assimilation in which
glucose is synthesized from carbon dioxi- de together with water. In photosynthesis reac- tions sunlight plays an important role. Photosyn- thesis
process in which the energy with low entropy carried by the sunlight is fixed proceeds as:
6H2O+6CO2+48hv(photon)-7C6H12O6+6O2
(Ec. 3)
In general, in the chloroplast of the plant, pho- tosynthesis can proceed by the light with the wa`velength of less than 700 [nm]. Namely in the
photosynthesis only a part of the visible light
of sunlight is used. This is because in order to make the absorption band of the chlorophyl into excited states it has to absorb such photons ha- ving
energy higher than certain value. In crea- ting one molecule of glucose, 48 photons are needed. In this photosynthesis reaction, appro- ximately 1/3 of
the energy of the photons (with lower entropy) is fixed in glucose. The rest is thrown away into the steam coming from the leaves, together with the
entropy generated by the irreversible processes. A plant is alive by throwing outside high entropy of waste by the transpiration of water.
Glucose and oxygen, being the products of the reactions have higher energies compared with those of the raw materials, i.e., carbon dio- xide and
water, since they have received the energy of light. Although the entropy of raw materials and that of the products do not change very much, the
products have higher energy than the raw materials. Thus the ratio of entropy to energy is comparatively low in the products’ case. The conversion
efficiency of energy is considerably high for such photosynthesis reac- tions.
In nature animals seek for energy with low entropy in glucose, which is synthesized by plants. The negentropy taken from vegetables and fixed partially
by herbivorous animals is used by carnivorouses. In this way the lowness of sunlight entropy is being used one by one through the food chain. Since
entropy is genera- ted additionally at each stage, smaller number of living organisms can be supported at later stages. A part of these living
organisms changed into fossil fuels such as coal and oil after their deathes. Thus, the lowness of sunlight entropy was fixed therein, though only
partially.
2. ENVIRONMENT AND ENTROPY
2.1 Earth is a Stationary Open System.
In the surface layer of Earth, various vital phe- nomena and social activities are taking place whereby entropy is created. Although entropy continues
increasing on Earth, we never reach to a "thermal death". The reason is that Earth, moving within the space of the solar system, receives heat and
light from the sun constituting an open system that discharges heat into space.
Such an open system maintains "non-equili- brium stationary state far from the thermal equi- librium one" by taking negentoropy from the environment
and, at the same time, throwing the generated entropy into the environment. By environment we mean the one extended from the surface of Earth and up to
the atmosphere. Since it throws entropy to space it constitutes a non-equilibrium stationary open system as well.
2.2 Circulation is Important in the Maintenance of Earth Environment as well as for Life.
In general there are two kinds of stationary open systems. One is a system wherein a simple diffusion process takes place. This type is called first
kind open system. The examples for this type are such as flows of falling down water, electric conduction and heat conduction. In this case the system
is locally almost equilibrium, and entropy does not increase any more. Such system is not related to any form of life.
Another one is a system where a circulation exists. Such system is called the second kind of open system, and is deeply related to life. In fact life
consists of various circulations. In the case of a human body various types of circulations such as blood circulation, lymph circulation, and
metabolisms of substances are functioning in mutual harmony.
While maintaining a stationary open system on Earth, circulation plays an important role as well. Three types of circulations can be men- tioned, i.e.,
the one of atmosphere, the one of water, and the one of living creatures.
2.3 How does Earth Maintains its Stationary Open System?
First of all, it exists an atmosphere kept by gravi- ty linked to the surface of Earth wherein the cir- culations of atmosphere and water take place
constantly by convection. The extra entropy is carried to the upper layer of the atmosphere by the convection of air together with thermal ener- gy
against gravity. Earth throws heat entropy to space as well. Water circulation is responsible for such processes. Heat entropy on the ground is
absorbed by the water evaporation. The steam rises to the upper layer of the atmosphere because it is lighter than air (the molecular
weight of water is smaller than that of nitrogen). Then heat is radiated to space together with en- tropy and the water returns back to the ground as
it rains.
On the other hand, matter entropy such as the one discharged by living organisms of Earth and corpses of living creatures are transformed into carbon
dioxide and steam, through decomposi- tion, by microorganisms such as bacteria, as well as by small creatures. These carbon dioxi- de and water are
reused for photosynthesis. That is the circulation of living organisms. Final- ly, the generated entropy of the abolition heat is radiated from clouds
in the atmosphere to space as infrared rays by water circulation and by the mechanism of entropy emission from the Earth. The reason why we can survive
in such an envi- ronment polluted by entropy is by virtue of the strong purification ability of such environment.
2.4 How Much Entropy is Thrown Away out of the Earth Sphere to Space?
Among the solar energy that reaches Earth, the thermal energy Q that remains on Earth surface is on average, approximately 77 [kcal] at 1
[cm2] per year [3]. Average temperature T1 on Earth surface is about 288 [K]. Therefore the entropy received by Earth from the Sun at
1 [cm2] per year is
Q
=266[cal/K]
T
1
(Ec. 4)
On the other hand, the residual heat Q is thrown away to space under an infrared ray form. The temperature T2 in the upper
layer of the atmosphere is about 250 [K], and therefore the entropy discharged out of the Earth sphere is
Q
=308[cal/K]
T
2
(Ec. 5)
Thus, the net entropy thrown by Earth at 1 [cm2 ] per year is
DS= Q- Q = 42 [cal/K]
T
1
T
2
(Ec. 6 )
through water circulation and convection in the atmosphere. This way Earth maintains a low entropy level. Of course, the above-mentioned was restricted
to the case of solar energy alone, and if we burn coal, oil, and nuclear fuels too, excessively, water circulation and convection in the atmosphere
cannot throw the amount of ex- cessive entropy, and thus entropy will increase in the Earth environment.
3. CONCLUDING REMARKS
In concluding we stress that the considerations based on the concept of entropy help us to understand life and the global environmental problems much
deeper. It is needless to say that the cause of present global environmental pro- blems is due to excessive economic activities of mankind. On
discussing such global environ- mental problems, the considerations from the energetic viewpoint alone are not sufficient and the ones based on the
entropic viewpoint are indispensable. The considerations on life from the entropic viewpoint are also useful in such field as food sciences.[4]
The concept of entropy is one of the most difficult for beginners of thermodynamics to understand, because of its abstract character. Adopting concrete
examples such as life and/or global environmental problems treated in this article may help to grasp this concept better and feel it more familiar, as
the one of energy.
REFERENCIAS
1. E.Schrödinger, What is life?–The Physical Aspect of the Living Cell–with Mind and Matter & Autobiographical Sketches, Cam- bridge
University Press, 1992.
2. Hayashi M. & K. Katsuura, Physics for Life Sciences (en japonés), Aichi Shuppan, 1998.
3. A. Katsuki, Basic Theory of Environment- Based on Physics (en japonés), Kaimei Sya, 1999.
4. Hayashi M., K. Katsuura, H. Vera Mendoza
& Pérez López, Tecnología y Sociedad, vol. 1, 1994.
![]()
![]() dE
in
=dE
out
dE
in
=dE
out