Introduction
Taenia solium (Ts) cysticercosis is a parasitic infection acquired by humans and pigs (the intermediary hosts) after ingesting the parasite eggs, released to the environment by human tapeworm carriers. Humans (the definitive hosts) acquire intestinal tapeworms by eating insufficiently cooked meat from cysticercotic pigs (Sciutto et al., 2000). A single tapeworm produces tens of thousands of eggs per day, which are shed to the environment (vegetables, running waters, soils) upon human tapeworm carrier open-air defecation practice. T. solium is endemic in most of the non-developed countries of Latin America, Africa and Asia, where all the conditions that favor transmission persist i.e., pigs rustically reared, inadequate and insufficient meat inspection and drainage and limited or no access to sanitary services (De Aluja, 2008).
In human, the most frequent form of the disease is Neurocysticercosis (NCC) that occurs when cysticerci (larval form of Taenia solium) are installed in the central nervous system (CNS).
1. Neurocysticercosis: A pleomorphic disease
NCC is an extremely pleomorphic disease (Coyle and Tanowitz, 2009; Fleury et al., 2010). In rural communities that present condition that favor transmission, it has been reported that almost 10% of the inhabitants have radiological lesions compatible with asymptomatic calcified parenchymal neurocysticercosis (Fleury et al., 2003; 2006). This is not surprising considering that immunological evidences support that more than 90% of the inhabitants have been in contact with the parasite (Chavarria et al., 2003). The absence of symptoms is expected since parenchymal NCC (P-NCC) is a benign form of the disease (Figure 1). In P-NCC the parasite tends to be destroyed probably by effective host immunity and in most cases evolves with mild or absence of symptoms. Epilepsy, the most important symptom in P-NCC, can generally be well controlled using anti-epileptic drugs (Duque and Burneo, 2017). However, it has been reported that the evolution can be improved by cysticidal treatment. Albendazole and Praziquantel are the drugs more extensively used for NCC treatment. It was shown recently that the use of a combination of ABZ and PZQ is more efficient for these patients than one of them used alone in case of multiple parenchymal parasites (Garcia et al., 2016).
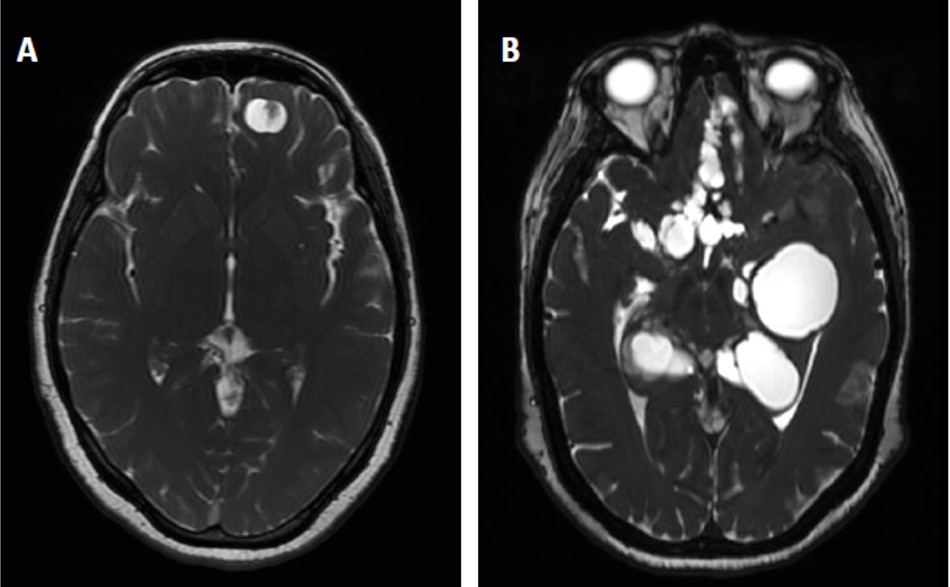 |
Figure 1. Radiological aspect of Neurocysticercosis caused by parenchymal (A) or extraparenchymal (B) cysticerci |
2. Extraparenchymal neurocysticercosis: a severe neurological disease
A completely different scenario, much more worrying, occurs when the parasite is installed in the cerebral extraparenchymal region (ExP-NCC), mostly in the subarachnoid space of the basal cisterns and in the ventricular system (Fleury et al., 2011). The Figure 1b illustrates this form of NCC, where multiple cysticerci occupy the basal cisterns. Probably, as in these locations parasites have more space to growth, their size at diagnosis is generally much higher than in other cerebral areas. Symptoms are more severe; particularly more of 70% of the patients developed intracranial hypertension due to the perturbation of the circulation of the cerebrospinal fluid (CSF) caused by the presence of the parasite (Marcin-Sierra et al., 2017). This situation can compromise the life of the patient and generally require the colocation of a ventriculoperitoneal shunt (Osorio et al., 2019).
3. Extraparenchymal neurocysticercosis: treatment
In 2017, Clinical Practice Guidelines for the Diagnosis and Treatment of NCC were published (White et al., 2017). Although this effort must be highlighted, it is evident that for the moment enough information are lacking to strongly recommend precise therapeutic scheme for extraparenchymal NCC (Carpio et al., 2018). Particularly, we can note that in these Guidelines, on the eleven recommendations made regarding treatment of extraparenchymal NCC (ventricular and subarachnoid), 6 of them (54.5%) have a low quality of evidence and a weak strength of recommendation (White et al., 2017). More clinical trials are urgent to made to determine with more strength and certainty the best management for these patients, as the problem is evident. Indeed, more than 50% of ExP-NCC patients are non-responders to the cysticidal treatment and require several cycles of albendazole to damage the parasite (Marcin Sierra et al., 2017; Osorio et al., 2019). ExP-NCC patients also have an exacerbated central inflammation that can be aggravated by increased antigen release with treatment-mediated damage to the parasite. This exacerbated inflammation is likely to damage the blood-brain barrier and promote antigen release that may induce in the periphery the production of regulatory cells to control it (Fleury et al., 2016). As the result we find in the blood and in the CSF of these patients an increase in Tregs accompanied by high levels of IL10 (Adalid-Peralta et al., 2012; Arce-Sillas et al., 2016). However, the central and peripheral increased of Tregs is not enough to control neuroinflammation and cysticidal drugs must be administered with glucocorticoids to avoid the inflammatory complications (mainly arachnoiditis and vasculitis) and intracranial hypertension (Toledo et al., 2018). Glucocorticoids (GCs), dexamethasone or prednisone, are the first-line drugs used for this purpose. However, with oral or endovenous administration only a low fraction of GC reaches the central nervous system (Meneses et al., 2019). Thus, high systemic doses are required for brain-specific targeting causing severe undesirable effects. To avoid this, alternative anti- inflammatory drugs like Methotrexate has been used (Mitre et al., 2007), in particular in diabetic patients. Other new therapies based on the use of antibodies against anti-tumor necrosis factor are also explored but it is not a realistic tool in non-developing countries (most of the endemic countries) considering the high costs of this therapy (Nash et al., 2019). The lack of experimental models of neurocysticercosis has limited the evaluation of possible immunomodulators that could be used for the finer control of neuroinflammation. Recently, a model of Ex-P-NCC in rats caused by the Taenia crassiceps cysticercus has been published. This new model will make it possible to carry out the research necessary to advance with new alternatives for the control of neuroinflammation in ExP-NCC (Hamamoto Filho et al., 2019).
Therefore, as seen, in these NCC forms we have a double therapeutical challenge: partial efficacy of the cysticidal treatment and non-optimal anti-inflammatory treatment. There is an urgent need to improve this current therapeutic approach. On the other hand, different evidences indicate that a certain degree of inflammation improve the effect of cysticidal drugs (Cárdenas et al., 2014; Toledo et al., 2018; Palomares-Alonso et al., 2020). Thus, we are in a complex situation, in which we must finely regulate the neuroinflammatory phenomenon, by reducing the elements involved in the generation of complications but by favoring those which participate in the success of cysticidal treatment (Toledo et al., 2018). A better understanding of the neuroinflammatory phenomenon and its relationship with the disease should make it possible to improve the current NCC therapeutic management. In particular, the central / periphery communication of the inflammatory phenomenon should make possible to find peripheral markers, which will be of great help in regulating the anti- inflammatory treatment
4. Conclusions and Perspectives
The treatment of ExP-NCC continues to be a challenge. Although there are drugs that in principle are efficient for the destruction of cysticercus, these do not work in an optima manner in a large part of cysticerci located outside the parenchyma. Evidences point that neuroinflammation should be regulated more carefully to increase the effectiveness of cysticidal treatment. To optimize the treatment of this severe form of NCC, future investigations should focus on finding peripheral markers to monitor the characteristics of the central inflammatory response, as well as developing effective anti-inflammatory drugs at low cost and with less collateral effects than the systemic GC currently available.
Acknowledgments
This study was supported by the “Programa para el desarrollo de vacunas, inmunomoduladores y métodos diagnósticos”, Instituto de Investigaciones Biomédicas de la Universidad Nacional Autónoma de México.
The authors have no conflict of interests.